SERVICES
SERVICES
We offer a complete array of services from concept strategy, reserach and development to contract manufacturing and licensing of skincare, nutraceuticals, and nutritional additives like collage, peptides, and amnio-acids.

R&D
LICENSING

CDMO

CMO
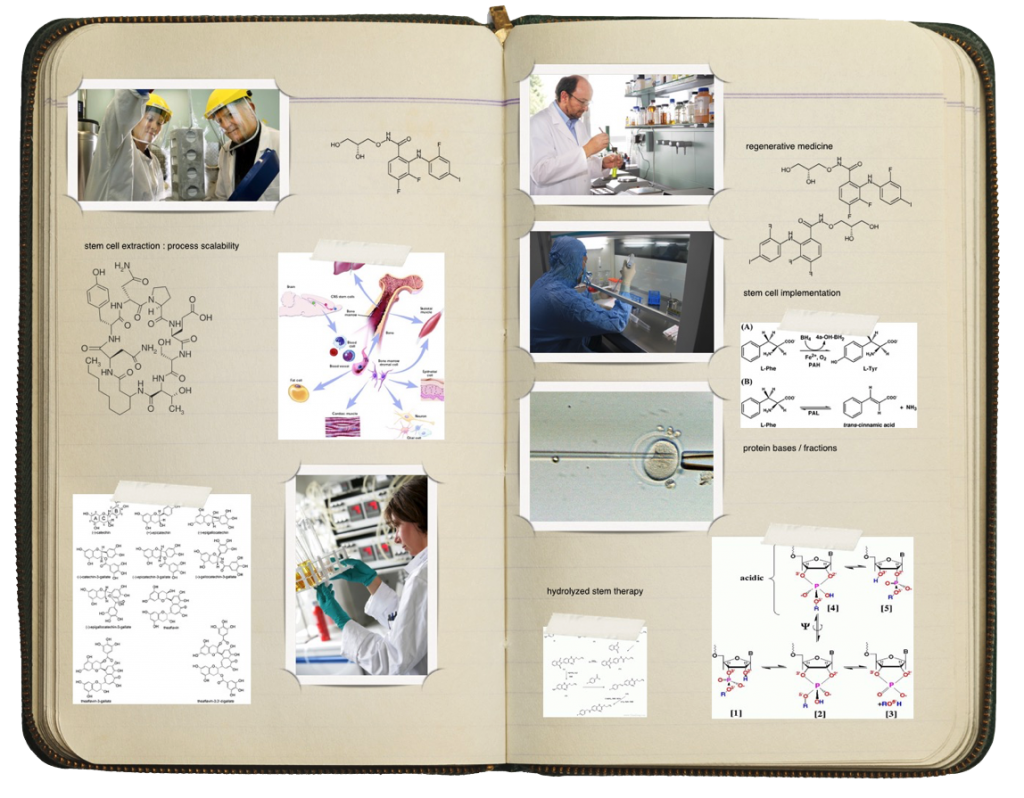
We offer a comprehensive research and development framework from concept, formulation, manufacturing, to commercialization of skincare, nutraceuticals, and nutritional additives like collage, peptides, and amnio-acids.
Our library of formulas and protocols is vast and robust. We are experts in our explicit ability to accelerate nutrient bioavailability to cells in the body. All product formulation, development, and manufacturing protocols entail lyophilization or hydrolization to unbind and free amino acids found in peptides derived from plants, or animal sources. This includes studies with accelerated stability (stress) studies, stability-indicating analytical method development, and other physiochemical characterizations designed to pinpoint potential product candidate stability problems and enable formulation optimization.
- Comprehensive biomarker of physicochemical properties
- Estimate a product’s stability when exposed to various common stresses.
- Establish stability assays to outline major degradation rates.
- Decide upon a lyophilized or hydrolyzed formulation for initial clinical studies.
- stabilizer, tonicity modifier; analytical methods; etc.
We encompass a robust variety of R&D services to help formulate your product:
- Analytical Methods
- Formulation Development
- Lyophilization Cycle Development
- Hydrolization Cycle Development
- In Use, Compatibility, and Robustness
- Technology & Innovation
- Modalities & Stability Studies
Via our state of the art facility we offer a complete and comprehensive variety of manufacturing services for nutraceuticals, dietary supplements, vitamins, proteins, and skincare. Our format includes liquid, soft-gel encapsulation, capsules, and powder. Our packaging and fulfillment solutions vary upon our clients’ requirements from bottles, blister pack, to sleeves.
Key Differentiators
- Vast formula and protocol library (500+)
- We are experts developing products target specific to a cell or group of cell requirements (transcriptome biomarkers)
- Experts in Hydrolization for rapid and high- grade nutrient bioavailability (free amino acids)
- Experts in Lyophilization for rapid and high- grade bioavailability (free amino acids)
- Experts in formulating and manufacturing products with hydrolyzed or lyophilized bovine or ovine placenta.
Our robust library of formulas and protocols affords us the ability to create and co-create new intellectual properties by way of licensing. Our IP strategy and monetization framework is comprised of three pillars:
- Limited Rights & Uses Of Our Formulas & Protocols In R&D Collaborations
- PRIVATE LABEL; the purchase of our stock formulas with your logo on the label
- Finalize a formulation development research protocol (matrix of buffer, pH, stabilizer, tonicity modifier; analytical methods; etc.)
- We encompass a robust variety of R&D services to help formulate your product:
- Analytical Methods
- Formulation Development
- Lyophilization Cycle Development
- Hydrolization Cycle Development
- In Use, Compatibility, and Robustness
- Technology & Innovation
- Modalities & Stability Studies
We offer seasoned acumen and expertise in both development and manufacturing, with advanced technological capabilities and the innovative protocols with agile business models to overcome development hurdles and speed your products to market, no matter what challenges may arise.
Our development and cGMP manufacturing capabilities are geared towards nutraceuticals, dietary supplements, vitamins, proteins, and skincare. Our format includes liquid, soft-gel encapsulation, capsules, and powder. Our packaging and fulfillment solutions vary upon our clients requirements from bottles, blister pack, to sleeves.
Our end-to-end process begins with in-depth analysis and strategic critical paths curtailed around your target requirements and product. Our library of formulas and protocols is vast and robust. We are experts in our explicit ability to accelerate nutrient bioavailability to cells in the body. All product formulation, development, and manufacturing protocols entail lyophilization or hydrolization to unbind and free amino acids found in peptides derived from plants, or animal sources. This includes studies with accelerated stability (stress) studies, stability-indicating analytical method development, and other physiochemical characterizations designed to pinpoint potential product candidate stability problems and enable formulation optimization.
- Comprehensive biomarker of physicochemical properties.
- Estimate a product’s stability when exposed to various common stresses.
- Establish stability assays to outline major degradation rates.
- Decide upon a lyophilized or hydrolyzed formulation for initial clinical studies.
Finalize a formulation development research protocol (matrix of buffer, pH,
- We provide end to end private labeling solutions from concept, to manufacturing, packing, fulfillment.
- We provide the finished formula in bulk client packages and fulfills.
III. TRANSFER OF TECHNOLOGY (TOT); we transfer our intellectual property to another organization (licensee) via a licensing agreement whereby the licensee attains a right to use the technology for a fixed period of time by paying a particular fee for it.